2023
April
Get the approval from the Ministry of Economic Affairs, R.O.C., to be listed as the Biotech and Pharmaceutical Companies
2022
December
BioNTech Announces Plans for a Clinical Trial Hub in Taiwan for mRNA-based Cancer Immunotherapies as Part of Asia-Pacific Expansion

November
Retain Biotech Signed a MOU with BioNTech
Retain Biotech Corporation Signed a Memorandum of Understanding with BioNTech to Support Clinical Trials in the Field of Oncology in East Asia

May
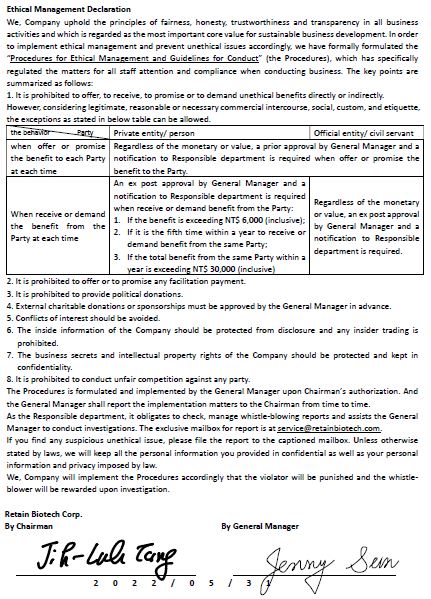
Ethical Management Declaration
We, Company uphold the principles of fairness, honesty, trustworthiness and transparency in all business activities and which is regarded as the most important core value for sustainable business development. In order to implement ethical management and prevent unethical issues accordingly, we have formally formulated the “Procedures for Ethical Management and Guidelines for Conduct” (the Procedures), which has specifically regulated the matters for all staff attention and compliance when conducting business.
2021
May
Successfully developed automated CAR-T manufacturing process
2020
September
Successfully developed CAR-T manufacturing process
2017
September
ISO:9001 Certified
August
Grand Opening
Peripheral Blood Immune Cell storage service
Peripheral Blood Stem Cell storage service


Offically Established
2016
November
Sign up contract with NTU
” peripheral blood stem cell and immune cell collection, freezing, and storage ” technique exclusively authorized.
March
Business registration
The Founding Years
2015
December
National Taiwan University Center of Industry-Academia Collaboration Council authorized the new cooperation (i.e. Retain Biotech Corporation) to be established.
The fist joint venture of NTU, Foxconn and YongLin Healthcare Foundation