Because of the successful clinical trial of CAR-T for relapsed refractory blood cancers, Science published a review of CAR-T as the greatest scientific breakthrough of the year in 2013. Several experts have also pointed out that immunotherapy will be the fifth most important treatment for tumors after surgery, chemotherapy, electrotherapy, and targeted drugs. US President Barack Obama responded to this major scientific breakthrough by including CAR-T technology as a key component of precision medicine in his 2015 proclamation to the American people, and by calling on and rewarding the Penn State medical team at the White House. The collaboration between Penn State and Novartis subsequently led to the first FDA approval of a "CAR-T cell" drug in August 2017, representing the beginning of an epoch-making cancer treatment.
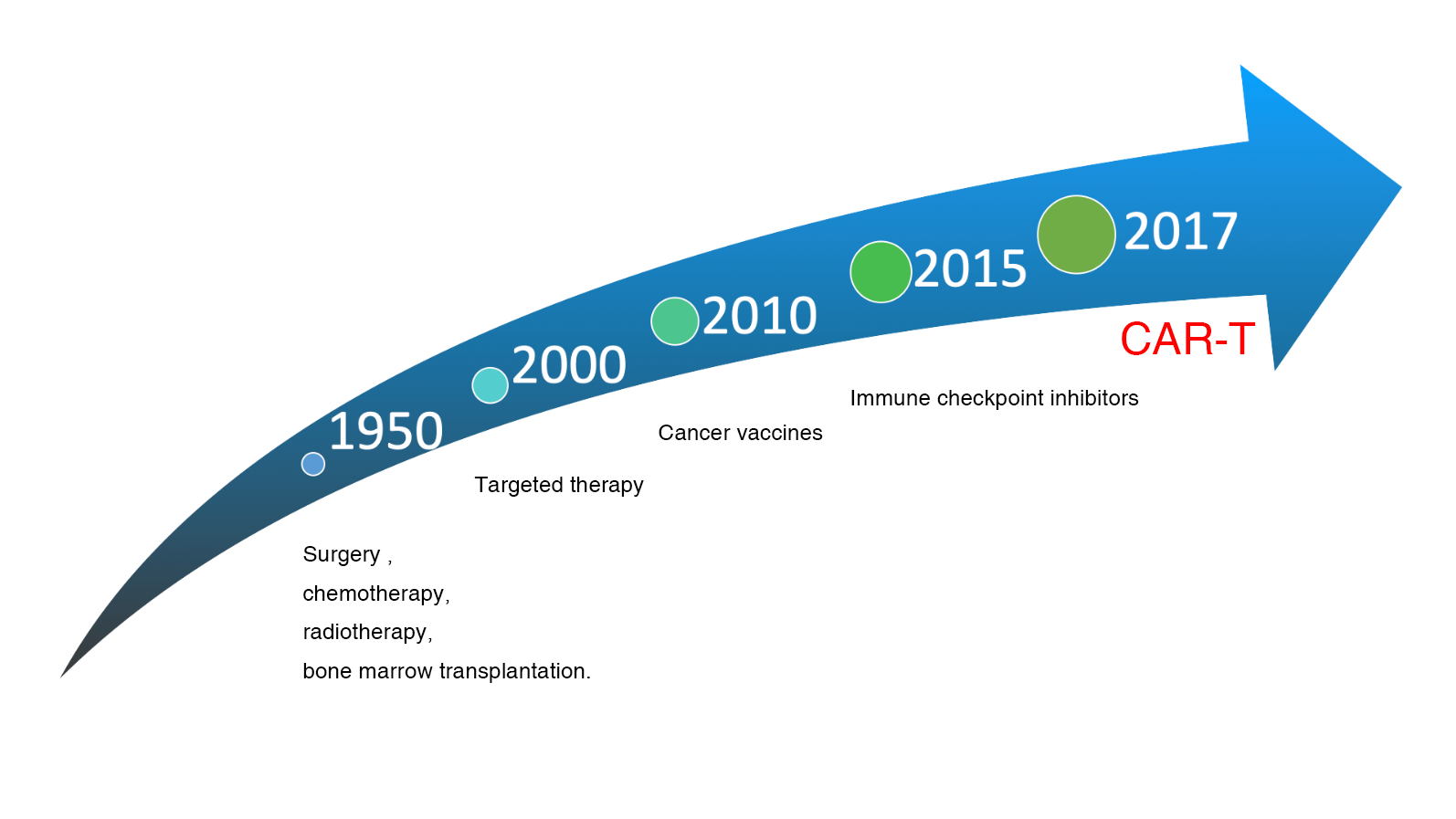
Since Penn State Carl June's research team has announced the success of CD19 CAR-T cells in treating patients with B-cell blood cancers that have failed to respond to conventional therapies, it was published in the New England Journal of Medicine in 2011. This breakthrough success ignited a worldwide fervor for CAR-T research. Not only academia such as Penn State University, Memorial Sloan Kettering Cancer Center, and National Cancer Institute, but also major pharmaceutical and biotech companies such as Novartis, Pfizer, Juno therapeutics, and Kite Pharma have all invested huge amounts of money and academia in CAR-T research. In particular, Juno and Kite, two companies focused on CAR-T with market capitalization of about NT$150 billion and NT$100 billion respectively, are competing to develop this highly technical and capital-intensive field of autologous CAR-T cell immunotherapy for cancer.
A New Milestone In Cancer Treatment
What is CAR-T?
CAR-T cells for tumor immunotherapy require high technology and the manufacturing process is quite complicated. Briefly, the patient's white blood cells need to be isolated at the first stage, the T cells are isolated and activated, the CAR-bearing virus is transduced to the T cells, and the CARs are expressed on the surface of the T cells after a few days, proliferate to the required "cell dose", and then the CAR-T cells are returned to the patient after the patient's immune function is reduced by chemotherapy.
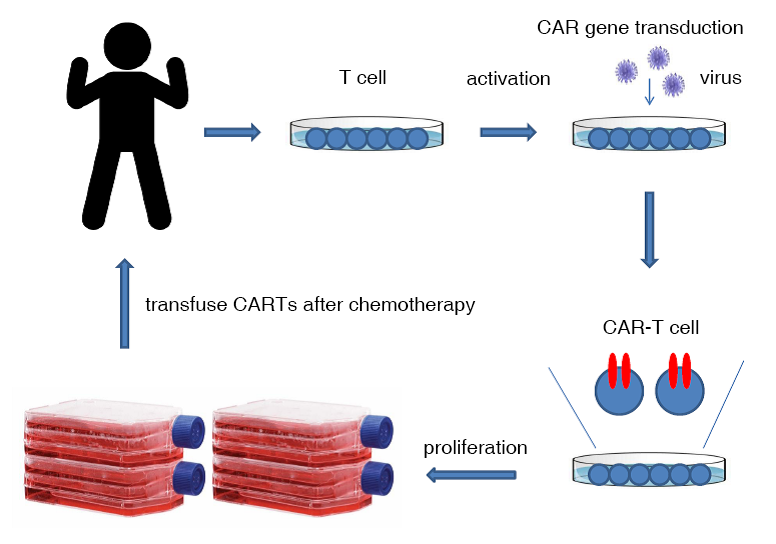
CAR-T Evolution
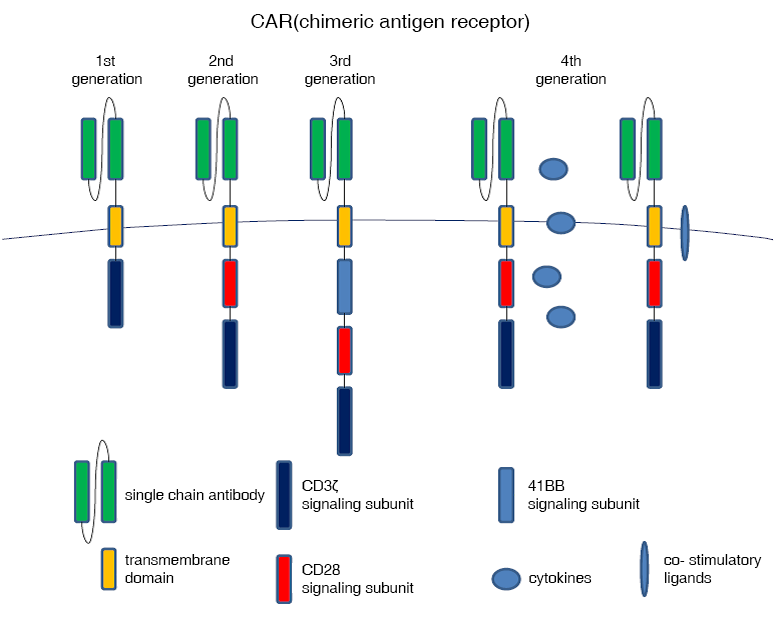
A breakthrough in CAR-T construction
Chimeric antigen receptors include an antibody-specific structural domain, mostly formed by combining the heavy and light chains of a single strain of antibody into a single-chain antibody with transmembrane and intracellular signaling structural domains. The first generation CAR contains a CD3ζ intracellular signaling domain. The second generation CAR adds a second intracellular signaling domain (e.g., 41BB or CD28), so that when CAR-T cells bind to an antigen, it triggers signals from two activated T cells, and the T cells can continue to be activated and not die. Several clinical trials have also shown that high concentrations of CAR-T cells can be sustained in patients for more than six months, leading them into long-term disease remission. Third-generation CARs add three intracellular signaling domains, and fourth-generation CARs add cytokines or costimulatory ligands to enhance the immune response to attack tumors.
Clinical Trial Of CAR-T Cells For Malignant Lymphoma
Published in the Journal of Clinical Oncology in 2015, this is the first clinical trial to successfully treat diffuse large B-cell lymphoma. In this clinical trial, four of seven patients (57%) with chemotherapy-naïve diffuse large B-cell lymphoma achieved complete remission after injection of autologous CAR-T cells. In three of these patients, remission was sustained for up to 22 months.
Clinical Trial Of CAR-T Cells For Solid Tumors
By replacing the single-chain antibodies of CAR-T with Tumor associated antigen (TAA) that can identify solid tumors, CAR-T cells can also kill solid tumors, and therefore various clinical trials of solid tumor CAR-T cells, such as EGFR, EGFRv3, HER2, mesothelin, IL13Rα2, CEA, GD2, CD133 and other tumor-associated antigens, have been launched in Europe and the United States. The results of the current clinical trials show that these specific TAA CAR-T cells respond to solid tumors. Therefore, a possible solution is to use two or more tumor-associated antigens - bispecific CAR-T cells to treat solid tumors to increase the efficacy, and the second is to use local injection of CAR-T or reduce the affinity between single-chain antibodies and TAA to avoid Thirdly, CAR-T cells expressing a protein such as an immune checkpoint inhibitor to avoid the dilemma of tumor microenvironment.